Overview
Priority Development Pipeline
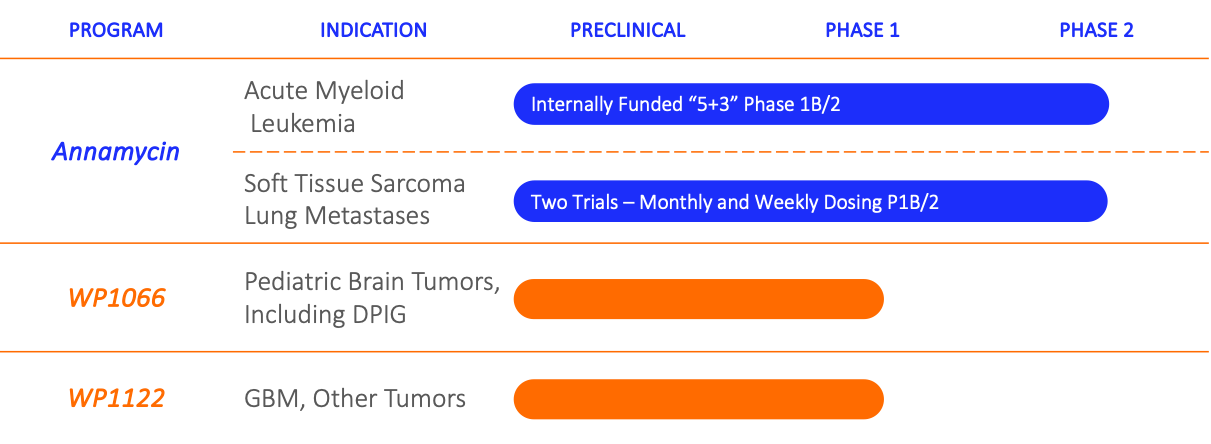
Your tooltip content goes here
Your tooltip content goes here
Your tooltip content goes here
Your tooltip content goes here
Priority Development Pipeline

Your tooltip content goes here
Your tooltip content goes here
Your tooltip content goes here
Your tooltip content goes here

