Better
Treatments,
More Tomorrows
Better
Treatments,
More Tomorrows
We Are
Advancing
a growing pipeline of product candidates for the treatment of highly resistant cancers and viruses.

Innovative
Therapies
through the use of proprietary technologies and data to develop a next generation anthracycline, immune/transcription modulators (STAT3 inhibitors) and metabolism/glycosylation inhibitors.
Innovative
Therapies
through the use of proprietary technologies and data to develop a next generation anthracycline, immune/transcription modulators (STAT3 inhibitors) and metabolism/glycosylation inhibitors.

The Annamycin Opportunity
Presented by CEO, Walter Klemp
The Moleculin Opportunity
A Three-Part Series Presented by CEO, Walter Klemp
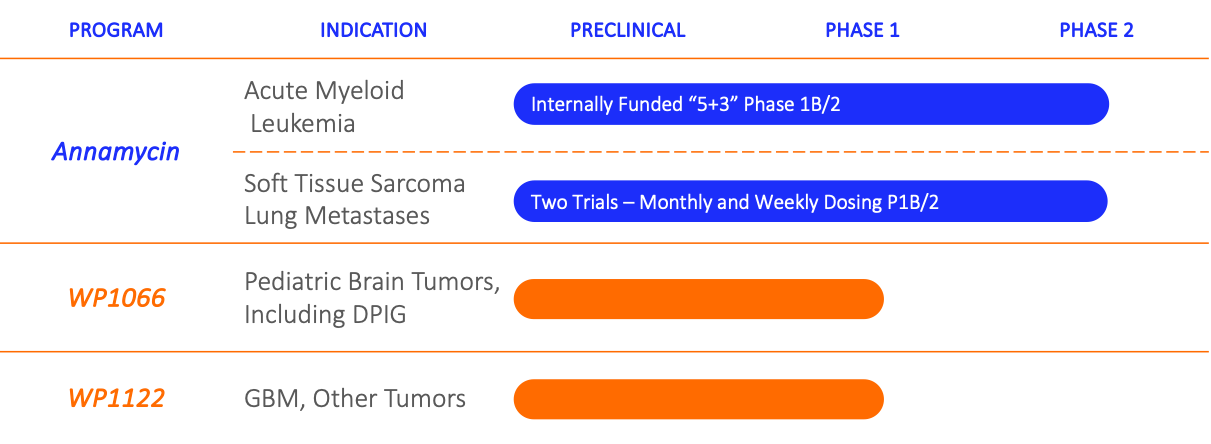
Priority Development Pipeline
AML Patient Journey and the Annamycin Opportunity
Latest Releases
Stock Information
NASDAQ: MBRX
NASDAQ: MBRX
Corporate
Presentation
Corporate
Presentation